On April 7, 2020, Prof. Zhi Hong’s lab from the Joint College of Edinburgh University and Zhejiang University together with Cong Yi’s lab from the Basic Medical College of Zhejiang University published a research article on Frontiers in Cell and Developmental Biology,titled "Mitochondrial Fusion Machinery Specifically Involved in Energy Deprivation-Induced Autophagy". This research expounded the relationship between mitochondrial morphology and energy deprivation-induced autophagy.

Main idea:
Autophagy is a process in which cells degrade intracellular substances for reuse when facing internal and external environmental changes. Autophagy can be classified into non-selective autophagy and selective autophagy according to different degradation substrates. Non-selective autophagy, commonly known as macroautophagy or autophagy, has no selectivity for degradation substrates. Selective autophagy relies on the interaction between specific autophagy receptors with LC3 / Atg8 to degrade damaged organelles. Numerous studies have shown that autophagy plays an important role in maintenance of cellular homeostasis and development of biological organisms. Dysfunctional autophagy contributes to various human diseases such as neurodegenerative diseases, typeⅡdiabetes, fatty liver, cancer etc.
Mitochondria, as the center of cell metabolism and signal transduction networks, regulate ATP synthesis, intracellular calcium homeostasis, oxidative phosphorylation and cellular apoptosis, and are one of the most important organelles for maintaining normal physiological functions of cells. Mitochondria vary in different cells and show dynamic changes. The dynamic balance of fusion and division affects the distribution and morphology of mitochondria, which in turn determines their physiological functions. Mitochondria form a dynamic network that is mainly regulated by mitochondrial fusion, fission, and tubulation machinery, which play an important role in maintaining the integrity of mitochondrial genetic material and DNA in living organisms. Mitochondrial damage will rapidly initiate mitophagy, but the link between mitochondrial morphology and non-selective autophagy remains poorly understood.
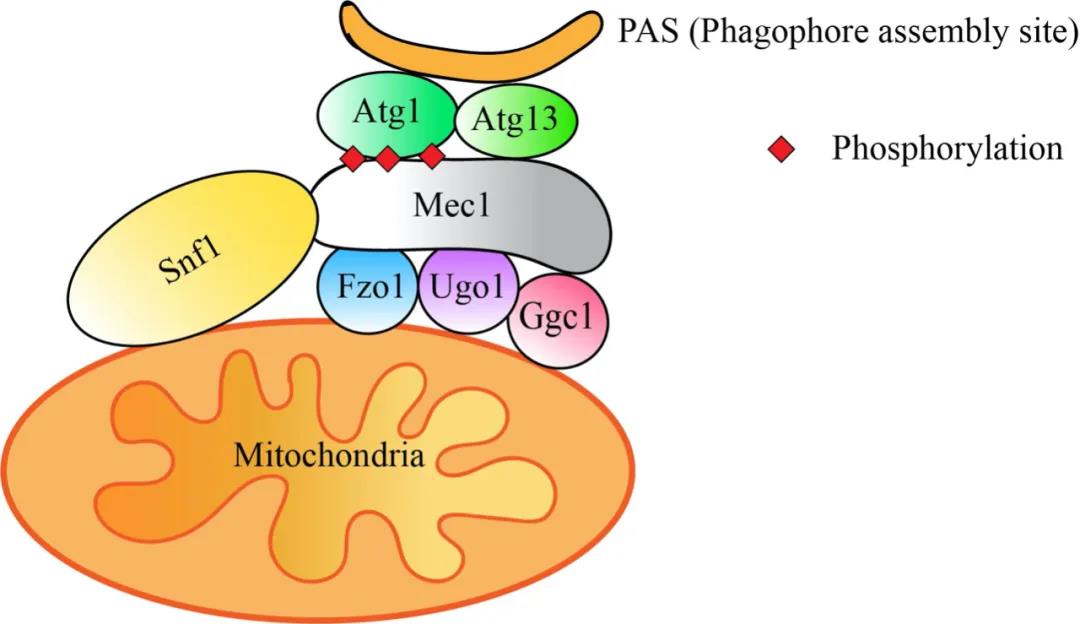
Drawn by Yuling Qin (PhD student in Prof. Zhi Hong’s lab)
In this study, they found that the mitochondria fusion machinery Ugo1, Fzo1, etc. regulate the interaction between Snf1 (AMPK homolog in yeast cells) and Mec1 (ATR homolog in yeast) by participating in aerobic respiration in an energy-deficient state. Depletion of Ugo1, Fzo1, etc. leads to a decrease in Snf1 activity and Mec1 phosphorylation level, which fails to recruit Atg1/ULK1 and other autophagic proteins to the pre-autophagosomal structure (PAS), therefore inhibits the occurrence of energy-deficient induced autophagy. Interestingly, the molecular machineries responsible for mitochondrial fission and tubulation are not involved in energy deprivation-induced autophagy.
Associate professor Choufei Wu and assistant researcher Weijing Yao are the co-first authors of this article. Researcher Cong Yi (left) and researcher Zhi Hong (right) are the co-corresponding authors of this article.
This research was supported by the National Natural Science Foundation of China and National Key R&D Program of China.
Lab info:
Zhi Hong’s research team has been focusing on spatiotemporal regulation of vesicle transport, organelle crosstalk network and cell homeostasis maintenance, and is keen to solve biological problems by using different disciplines. If you are also interested in freely exploring and risk taking, welcome to join us!
E-mail: zhihong@intl.zju.edu.cn
Home page of Prof. Zhi Hong: https://person.zju.edu.cn/hongzhi







