Bats are the only mammals capable of powered flight, and as an evolutionary adaptation to the metabolic stress of flight they have developed a unique tolerance to stress. This links to their inherent viral tolerance, as many stress-byproducts mimic viral PAMPs. Bats exhibit tolerance to pathogenic viruses such as Ebola and SRAS yet why these bats are truly tolerant and how much variation there is between bats remains to be fully explored.
As the COVID-19 pandemic developed, we produced reference-quality long-read genomes of twelve new bat species, including Rhinolophid Chinese horseshoe bats, the hosts of SARS-like viruses. A comparative cross-species analysis across 115 mammal species, using 17,000 orthologues, confirmed unique evolution of the “immune system process” in bats, with a different strength of selection observed in different branches of the phylogenetic tree.
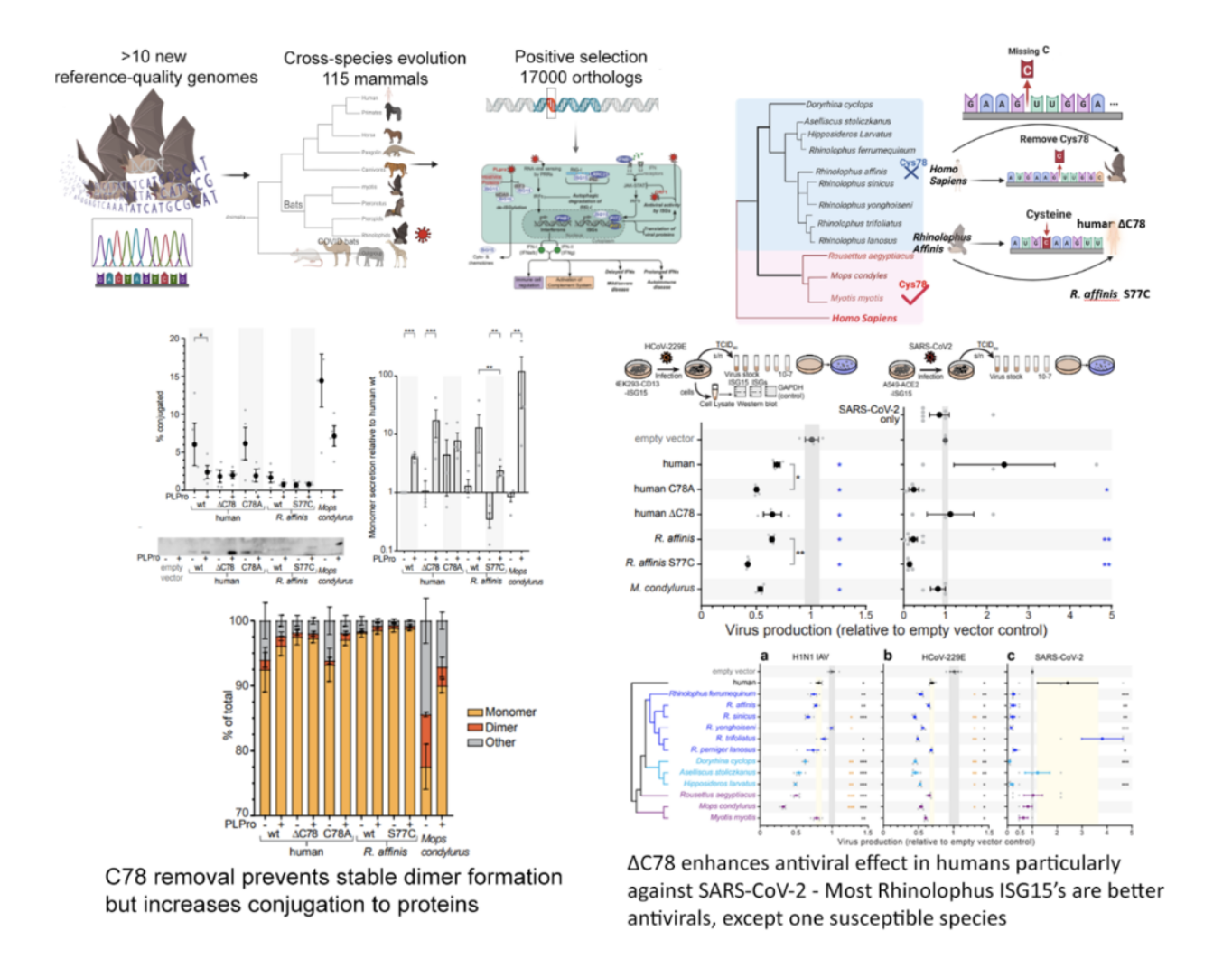
Adaptive evolution identified genes under positive selection in Chinese horseshoe bats related to viral infections, host cell entry, innate immune regulation, complement activation, and immune cell signaling. One gene identified includes ISG15, which displays a unique cysteine deletion only observed in Rhinolophus and Hipposideros bats, the hosts for SARS-Like viruses. This same Cys78 residue in humans forms a disulfide bond, stabilizing the ISG15 dimer for secretion and is so implicated in driving hyper-inflammation in humans.
Our experimental validation revealed an additional antiviral efficacy inside cells for bat ISG15, identifying virus- and species-specific differences. We show a similar antiviral capacity to human for Influenza A virus but an enhanced antiviral effect against coronaviruses, including SARS-CoV-2, which was seen more strongly for horseshoe bat ISG15’s. Human ISG15 is a poor inhibitor of SARS-CoV-2, as it is cleaved by SARS-CoV-2’s PLPro enzyme. Horseshoe bat ISG15 on the other hand, with the exception of one species, can bypass PLPro cleavage and functions to strongly block SARS-CoV-2 production. ISG15 functions in a manner similar to ubiquitin, being conjugated to other proteins and regulating the cellular responses. Bat ISG15 shows enhanced intracellular ISGylation in the presence of virus. Thus, ISG15 could be one of the factors that contribute to the ability of rhinolophid and hipposiderid bats to launch effective antiviral responses without triggering excessive inflammation.
Our evolutionary analysis highlights the importance and necessity of having multiple reference genomes available for functional validation studies in immunity and shows species-specific phenotypes even amongst closely related bat species. We identified Rhinolophid-specific evolutionary signatures in immune activation, identifying several targets involved in antiviral immunity. The greater impact of this modulation in the context of human immunity is being further explored.
Yue Dong, co-first author, is a University of Edinburgh (Haining) PhD student. Xioameng Li is a final year Zhejiang University-University of Edinburgh (dual-degree) PhD student. Ping Lu and Yixin Yang were Zhejiang University-University of Edinburgh undergraduate students during this work. Ping Lu has stayed on for her PhD. A. Professor Aaron Irving is co-corresponding author together with his collaborator, Michael Hiller from Senckenberg Research Institute, Frankfurt Germany. Many other authors consist of members of the Bat1K genome consortium.







