Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer that accounts for 15–20% of all cases and is characterized by early onset, rapid progression, and high metastatic potential. Due to the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), TNBC offers limited therapeutic options. The primary treatment of TNBC patients is chemotherapy due to the high chemosensitivity of TNBC tumors. However, rapid recurrence and drug resistance often result in poor outcomes. Although immune checkpoint blockade (ICB) therapy has shown promise, its clinical efficacy in advanced and metastatic TNBC remains unsatisfactory. Therefore, developing a strategy that integrates precise drug delivery, tumor microenvironment (TME) modulation, and multimodal therapy is urgently needed.
Recently, the research team led by Dr. Wenwen Huang from the Zhejiang University–University of Edinburgh Institute published a research article in Advanced Science, reporting a genetically engineered light-responsive in situ hydrogel system for immunomodulation and multimodal therapy of metastatic TNBC. The team designed a family of cysteine-tagged silk-elastin-like proteins (cSELPs) through synthetic biology and rational protein design strategies. These tri-block chimeric proteins integrate silk-inspired crosslinking motifs, elastin-based thermo-responsive motifs, and a photothermal agent-binding motif to form a biocompatible and stimuli-responsive protein hydrogel platform.
As shown below, the cSELP hydrogel precursor solution undergoes in situ gelation at the tumor site, forming a stable therapeutic reservoir. Genetically introduced cysteine tags bind to gold nanorods (AuNRs) through strong Au–S bonds, eliminating the need for toxic surfactants and achieving stable photothermal conversion under near-infrared (NIR) irradiation. This enables precise and on-demand drug release while maintaining excellent biocompatibility. Upon NIR light exposure, the system induces mild photothermal therapy (PTT) and facilitates the coordinated release of the chemotherapeutic drug doxorubicin (DOX) and the immune checkpoint inhibitor anti-PD-L1 (aPD-L1). Through synergistic “photothermal–chemo–immuno” therapy, the hydrogel promotes immunogenic cell death (ICD), enhances T-cell infiltration, and activates systemic immune responses, effectively converting immunologically “cold” tumors into “hot” ones.
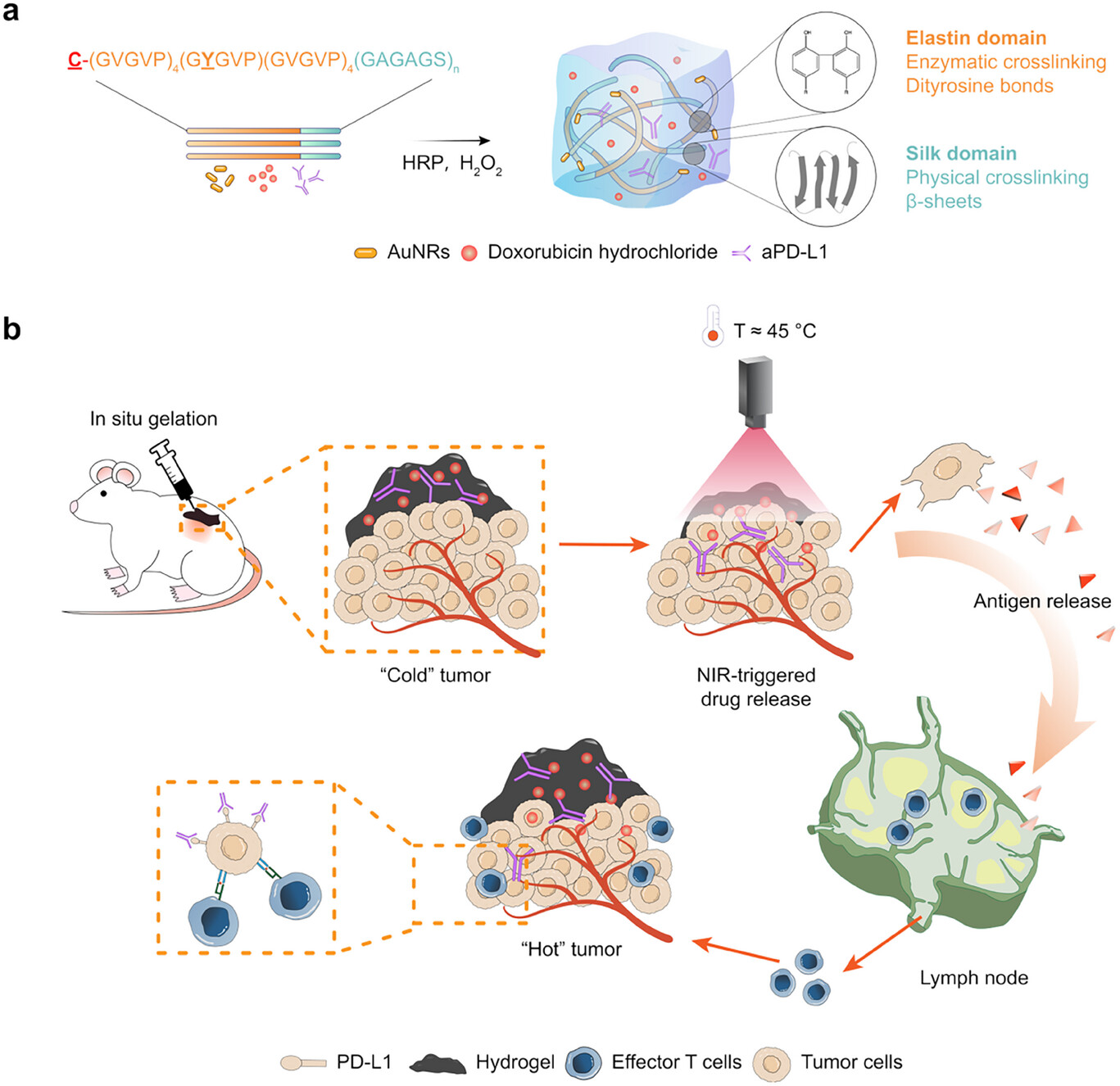
In vivo, the c2Y@Au/DOX/aPD-L1 hydrogel achieved simultaneous suppression of both primary and distal tumors while exhibiting excellent biocompatibility and long-term safety. Compared to monotherapies, the system achieved strong antitumor efficacy with low drug doses and short irradiation time, and minimal systemic toxicity. In addition, the hydrogel demonstrated remarkable antibacterial performance, broadening its potential applications to post-surgical infection control and wound healing.
In summary, Dr. Huang’s team developed a versatile, controllable, and expandable light-responsive hydrogel platform by integrating genetic engineering and materials design. This work offers a new strategy for treating metastatic TNBC through precise locoregional delivery and synergistic immunomodulation, showcasing broad potential for clinical translation.
Authorship and Acknowledgments
Xinchen Shen (dual-degree Ph.D.) is the first author. Jiajia Zhang (M.Sc.), Ziheng Bai (currently a Ph.D. student under Dr. Angelica Foggetti at ZJE), Junhan Ou (Ph.D. candidate), and Kaiyue Zhang (Ph.D. candidate) from Dr. Huang’s group, and Tongyan Liu (M.Sc.) from Dr. Qianting Zhang’s group at ZJE are co-authors. Dr. Kaixiang Zhu from the Second Affiliated Hospital of Zhejiang University School of Medicine, as well as Dr. James Q. Wang, Dr. Chaochen Wang, Dr. Qianting Zhang, and Dr. Linrong Lu from ZJE, made important contributions to the study. Dr. Wenwen Huang (Zhejiang University–University of Edinburgh Institute) is the sole corresponding author. This research was supported by the National Natural Science Foundation of China and the Zhejiang Provincial Natural Science Foundation.
Article Link: https://advanced.onlinelibrary.wiley.com/doi/10.1002/advs.202512355







