A collaborative study led by Dr. Heng-Jia Liu's team at the Zhejiang University-University of Edinburgh Institute (ZJE) and Professor Elizabeth Henske's group at Harvard Medical School has been published in European Respiratory Journal (one of the top-tier respiratory medicine journals), under the title *"Modulation of infiltrating CD206-positive macrophages restricts progression of pulmonary lymphangioleiomyomatosis.". The research team pioneered an integrated approach combining single-cell RNA sequencing, spatial transcriptomics, and macrophage-LAM cell coculture systems with clinical sample analysis. Their groundbreaking work provides the first evidence that CD206-positive immunosuppressive macrophages play a pivotal regulatory role in the progression of pulmonary lymphangioleiomyomatosis (LAM). The study demonstrates that targeted modulation of these macrophages using CD206-binding peptide (RP-182) effectively suppresses disease progression, establishing a novel immunotherapeutic intervention strategy for LAM treatment.
Lymphangioleiomyomatosis (LAM) is a rare cystic lung disease that almost exclusively affects women of reproductive age. It is essentially a low-grade neoplastic condition characterized by the proliferation of abnormal smooth muscle–like cells harboring TSC1 or TSC2 mutations. These cells infiltrate lung tissue, disrupt alveolar structures, and lead to clinical symptoms such as dyspnea, spontaneous pneumothorax, and chylothorax, eventually progressing to respiratory failure. Although mTOR inhibitors are currently approved for LAM treatment and can slow disease progression, symptoms typically recur upon discontinuation, and there is no curative therapy. The immune system plays a crucial role in the progression of many neoplastic diseases, with immunosuppressive M2-like macrophages known to promote tumor progression in various malignancies. However, the role of M2-like macrophages in LAM and their potential as therapeutic targets remain poorly understood.
https://publications.ersnet.org/content/erj/66/5/2500084

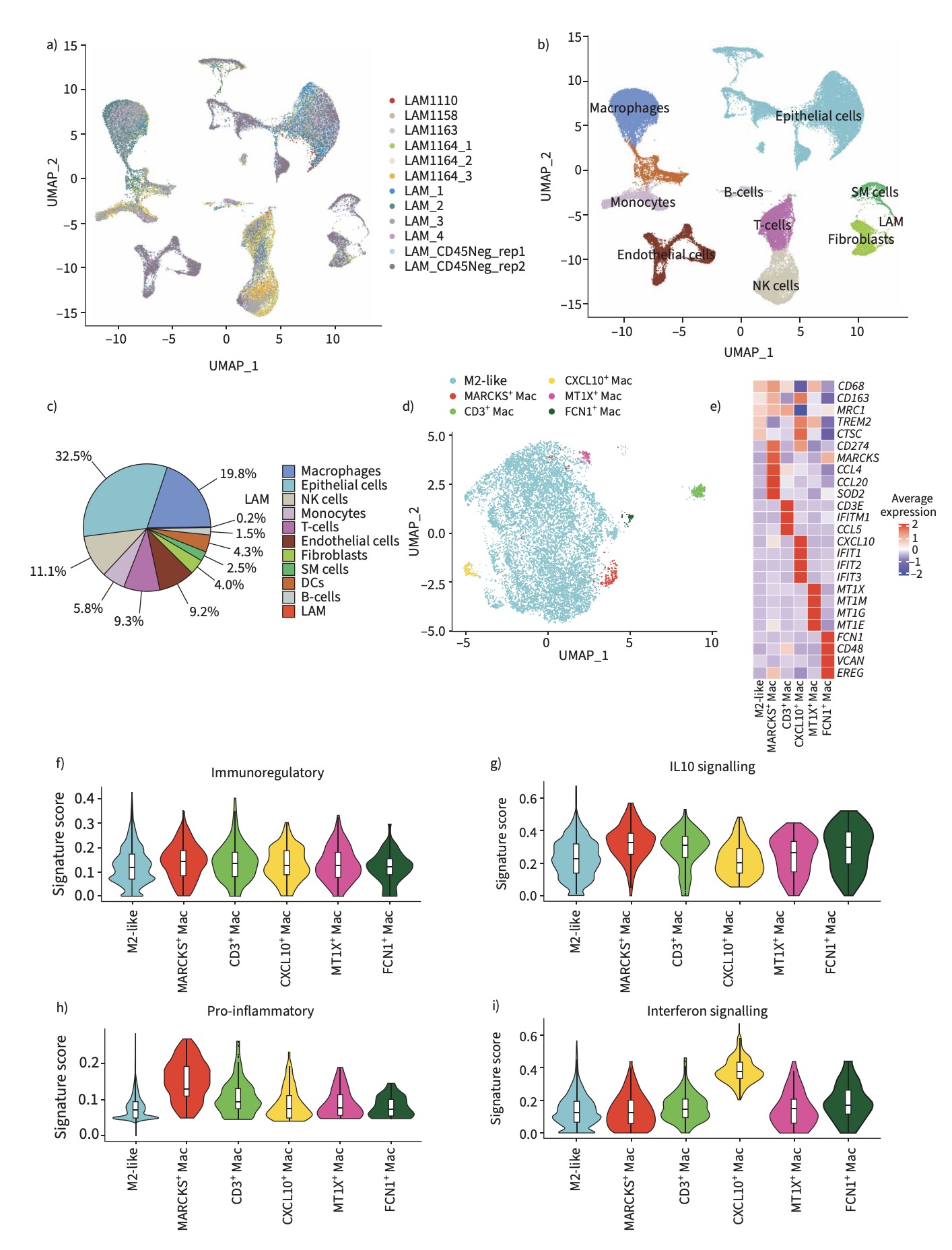
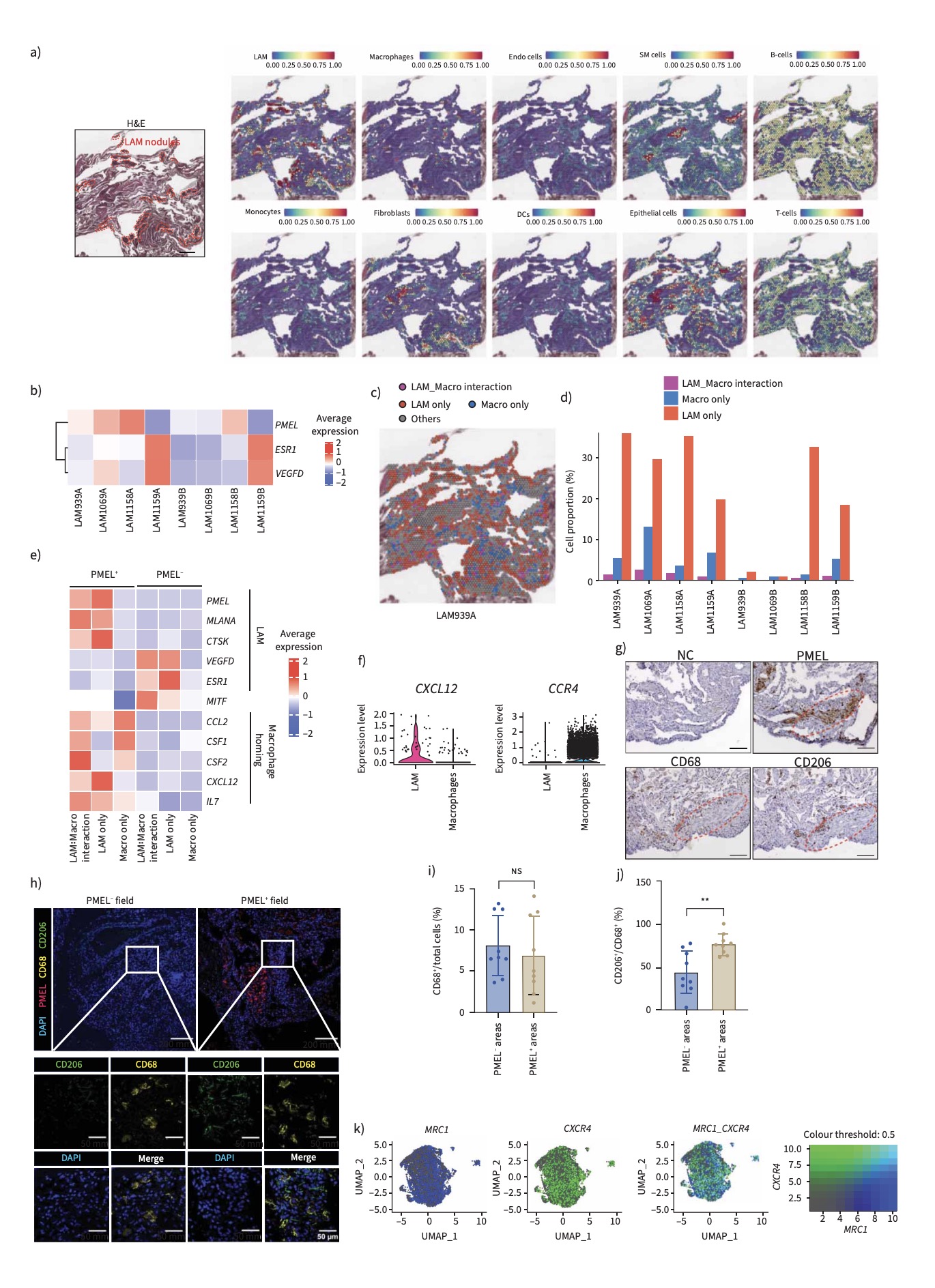
To elucidate the immune landscape of LAM, the research team constructed a single-cell transcriptomic atlas of human pulmonary LAM tissues. High-throughput single-cell RNA sequencing revealed that most macrophages within the lesions exhibit immunosuppressive M2-like characteristics, expressing canonical markers such as CD206 (MRC1) and CD163. Spatial transcriptomics and immunofluorescence analysis showed that M2 macrophages were frequently located adjacent to LAM cells, which highly expressed the chemokine CXCL12, a macrophage recruitment factor. These results suggest that LAM cells may establish a pro-tumor microenvironment by actively attracting and reprogramming macrophages into an M2-like state.
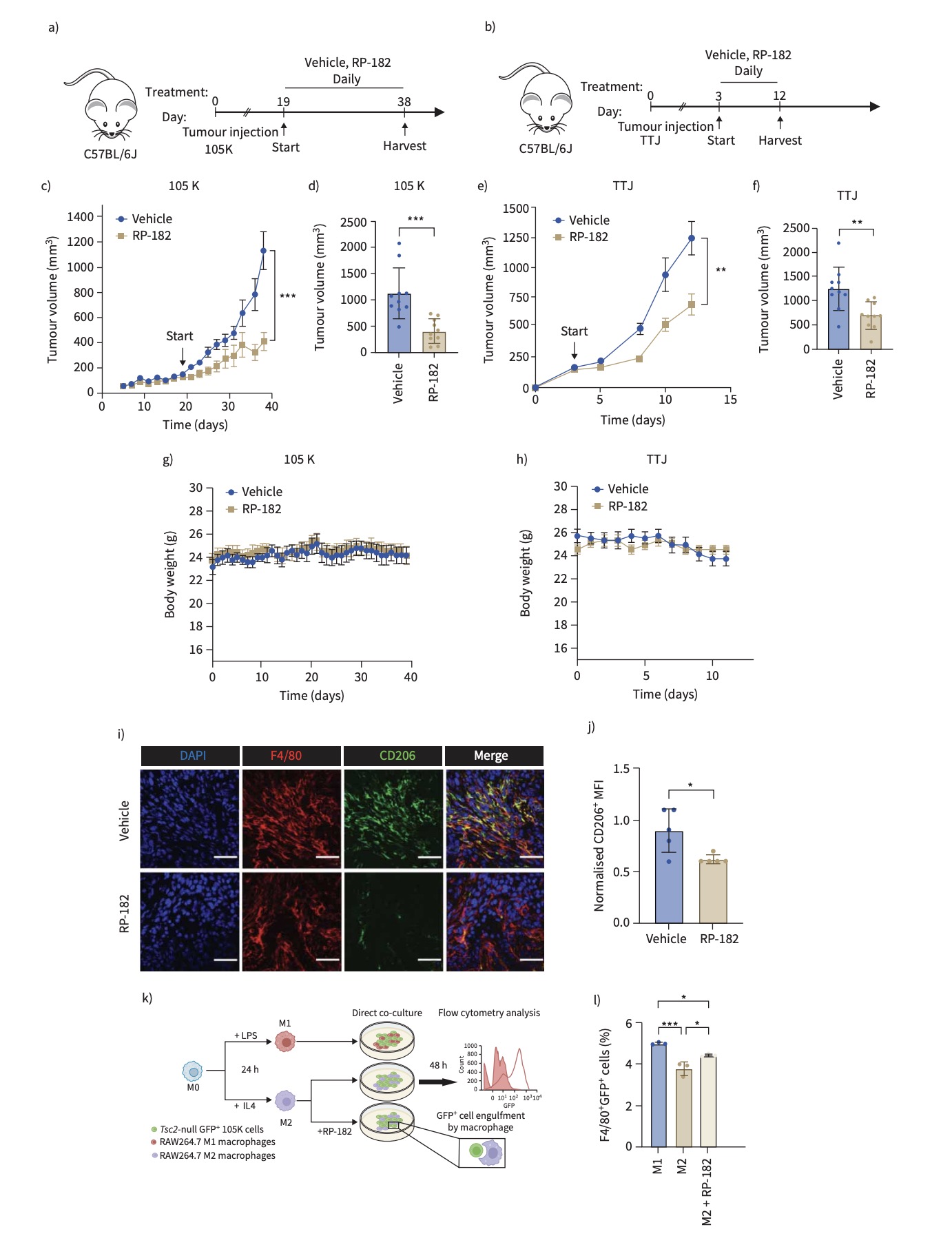
To further explore this mechanism, the team employed direct co-culture models of TSC2-deficient cells with either human or mouse macrophages. They found that macrophages upregulated M2-associated markers under the influence of TSC2-deficient cells. Moreover, in a subcutaneous TSC2-null tumor mouse model, the team evaluated the therapeutic potential of RP-182—a synthetic peptide that reprograms macrophages toward an anti-tumor M1 phenotype. RP-182 treatment significantly impaired tumor growth, highlighting a novel immunotherapeutic approach for LAM.
In summary, the study reveals that LAM cells recruit macrophages through CXCL12 signaling and promote their polarization into M2-like, immunosuppressive cells, creating a microenvironment conducive to disease progression. This research represents the first attempt to target M2-like macrophages in LAM using RP-182, establishing proof-of-concept for a new therapeutic strategy. The findings not only demonstrate a significant tumor-inhibitory effect in vivo but also offer fresh perspectives for developing immune-based therapies for this currently incurable disease. The study deepens our understanding of the LAM immune microenvironment and lays the groundwork for future precision immunotherapies targeting macrophage phenotypes.
Dr. Heng-Jia Liu, Principal Investigator at the ZJU-UoE Institute, is the first author and corresponding author of the paper. Bo-Xiang Wu (undergraduate, Class of 2021) and Yue Jin (graduate student, Class of 2024), both from Liu’s team, made substantial contributions to single-cell transcriptomics and spatial transcriptomic analyses as well as manuscript preparation and revision.
Heng-Jia Liu’s team has long focused on rare tumors driven by hyperactivation of the mTORC1 signaling pathway, including tuberous sclerosis complex (TSC) and lymphangioleiomyomatosis (LAM), with an emphasis on impaired immune responses and targeted therapeutic strategies. By integrating single-cell and spatial proteomics, transcriptomics, and preclinical models, the team has systematically uncovered immunosuppressive mechanisms within the tumor microenvironment. To date, the group has published more than 20 papers as first or corresponding author (including co–first and co–corresponding) in journals such as European Respiratory Journal, Nature Communications, Chest, and PNAS. The team leads a National Natural Science Foundation of China (NSFC) Young Investigator grant, has been selected for the Zhejiang Province Young Talent Program, and holds academic appointments including Honorary Lecturer at the University of Edinburgh.







